Dr. Gross performs multiple types of joint replacement including revision surgery. We monitor all of our patient’s results long-term. The American population is highly mobile, and 80% of our patients come from outside of South Carolina. However, we still manage to maintain up-to-date follow-up in 96% of cases (many of our patients do not feel the need to follow-up since they have excellent results with no pain; even if this is the case, we still recommend routine follow-up). We periodically update results for the most common procedures performed.
Survivorship Curves: We use the Kaplan-Meier method. This takes into account patients being lost to follow-up as well as deaths due to unrelated causes. Each failure is time-weighted by this method. The resulting curve provides the odds of an implant still being in place in the patient at any chosen time point from surgery. Joint replacements are not permanent. The longer that you follow a group of patients, the higher the failure rate that you record. It is very difficult to compare previous results to more recent cases because of the difference in follow-up. However, if techniques improve, the survivorship curve of the latest group of patients will be higher and flatter than the previous group. The following results are some of the best in the world; see our publication section for comparison of these results to other surgeons.
1. Hip Resurfacing
Updated 7/2025
In my (Dr. Gross’) opinion, Hip Resurfacing Arthroplasty (HRA) is the best way to reconstruct a severely arthritic hip. It is more complicated to perform than a standard Total Hip replacement (THR); therefore, few surgeons are willing to offer this procedure. In the major joint registry reports, THR has better implant survivorship in most groups of patients (except in men with osteoarthritis who are under 60 years old).
However, registries measure outcomes for average surgeons. The average surgeon performs less than 2.5 HRA cases/year. This is not adequate to be an expert. In reports by high-volume hip resurfacing surgeons, results are much better than the registries suggest.
Dr. Gross has now performed over 6800 Hip Resurfacing Arthroplasty (HRA) procedures over the last 20 years and currently performs nearly 500 cases/year. The proven advantages of HRA are better function, longer implant survivorship, fewer dislocations, no thigh pain (from a THR stem), bone preservation, and longer life expectancy than THR patients.
HRA does not result in a normal hip. But, when done by an expert, it more nearly approaches a normal hip in biomechanics and function and patients are more likely to resume heavy work and impact sports than they could with a THR. Long-distance running is even possible for many (but not all) patients. Also, activities that require an extreme range of motion such as full squats, yoga, gymnastics, and ballet are possible because HRA has near-normal stability.
There are several other HRA surgeons in the world who have reported similar long-term implant survivorship data. There is only one single-surgeon report of ceramic-ceramic THR from Korea that can match the results reported here. Most failures occur during the first two years after surgery, which is why it is critical to severely limit activities in the first 6 months to allow adequate healing. After that, a patient can gradually return to completely unrestricted activity. There remains a slow rate of failure that occurs over time. But this does not seem to be affected by activity.
Therefore, the overall failure rate increases for a group of patients as the length of follow-up increases. Herein, we report implant survivorship, for all three of our HRA implant groups (we no longer use Corin or Biomet hybrid implants; we exclusively use Biomet uncemented implants). Not all complications lead to failure.
Below is a complete list of ALL major complications (not just failures/causes for revision) in the >5500 HRA cases performed using the Biomet uncemented system between 2007 and 2023. This allows a minimum of 2 years follow-up.
Group I: Failures (requires revision surgery) – TOTAL: 63/6202 (1.0%)
-
Cause of Failure/Revision
-
# cases
- Femoral neck fracture
- 19
- Failure of acetabular ingrowth
- 11
- Adverse-wear related failure
- 4
- Femoral head collapse (osteonecrosis)
- 3
- Late acetabular loosening
- 6
- Component Shift
- 2
- Late Fracture
- 6
- Early Infection
- 0
- Unknown Cause (revised elsewhere)
- 3
- Recurrent Instability
- 2
- Unexplained Pain
- 2
- Late Infection
- 2
- Psoas Tendonitis
- 1
Group II: Complications (requires reoperation*) – TOTAL: 36/6202 (0.6%)
*implants are not removed during reoperation
-
Cause of Reoperation
-
# cases
- Late Fracture ( > 6 months)
- 11
- Early Fracture ( < 6 months)
- 2
- Early Infection (cured)
- 4
- Hematoma
- 4
- Fascia Failure
- 3
- Other
- 4
- Dislocation
- 1
- Abductor Tear
- 1
- Acetabular Cup Shift
- 1
- Psoas Tendonitis
- 1
Group III: Other complications (conservative treatment) – TOTAL: 138/6202 (2.2%)
-
Complication
-
# cases
- Acetabular component shift (nonsymptomatic)
- 31
- Dislocation
- 20
- Cardiovascular complication
- 18
- Nerve Palsy/Injury
- 8
- Urinary Retention
- 8
- Spinal Headache
- 13
- Other
- 8
- Hematoma
- 5
- Early Fracture ( < 6 months)
- 5
- Late Fracture ( > 6 months)
- 4
- Femoral Component Shift
- 4
- Anxiety Attack
- 3
- GI Bleed
- 2
- Nausea/Vomiting
- 2
- Unexplained Pain/Swelling
- 3
- Severe Constipation/Diarrhea
- 2
- Abductor Tear
- 2
- Wound Dehiscence
- 1
- Early Infection
- 1
- Fascia Failure
- 1
- Other
- 8
Implant Survivorship – Includes ALL implant types*: 7000 cases over 20 years
*unless noted otherwise in each graph
Survivorship of hip resurfacing continues to improve as we gain more experience and identify measures to prevent failures. These survivorship curves give the reader an opportunity to see what the odds are that their implant will still be functioning at some time point after implantation.
We present three Kaplan-Meier survivorship curves: all implant groups, Biomet implants grouped by age, and Biomet implants grouped by sex. Unlike THR, HRA survivorship does not vary by age (overall 99.1% 16-year implant for both age groups).
Most failures occur in the first 1-2 years. If you make it to one year, your implant survivorship at 13 years is 99.6%. If you make it to 2 years, it is 99.8%. Dr. Gross' uncemented resurfacing implant survivorship beats all registry benchmarks for THR regardless of age or sex.
In our recent multicenter international study (27 HRA centers in 13 countries), over 11,000 cases in patients under age 50 with multiple different metal-on-metal HRA brands showed a 90% 20-year implant survivorship (93% in men and 81% in women). For comparison, THA registries show approximately 80% implant survivorship at 10 years and 50% at 20 years in this age group.
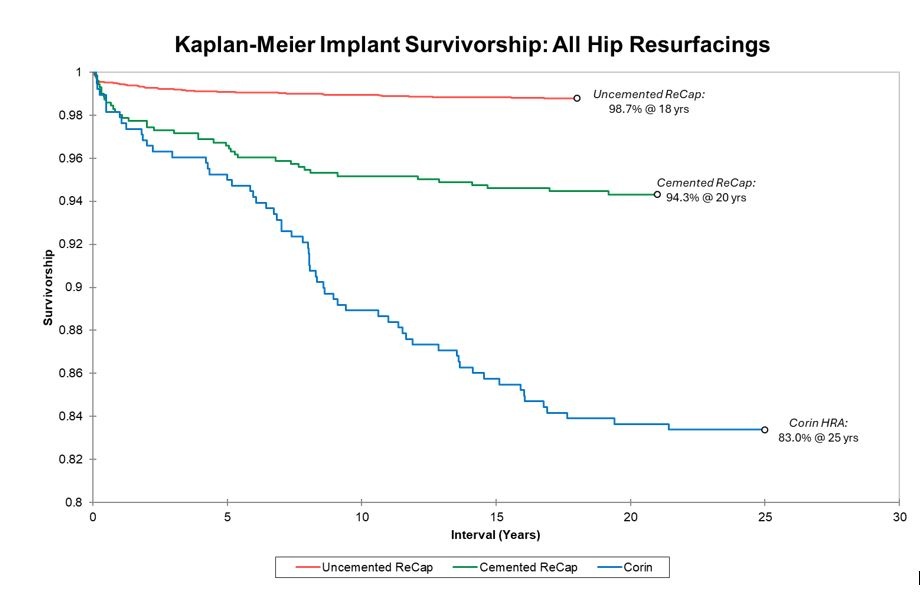
Note that the survivorship y-axis begins at 80%.
There have been no instances of adverse metal wear from any surgeries performed after 2009.
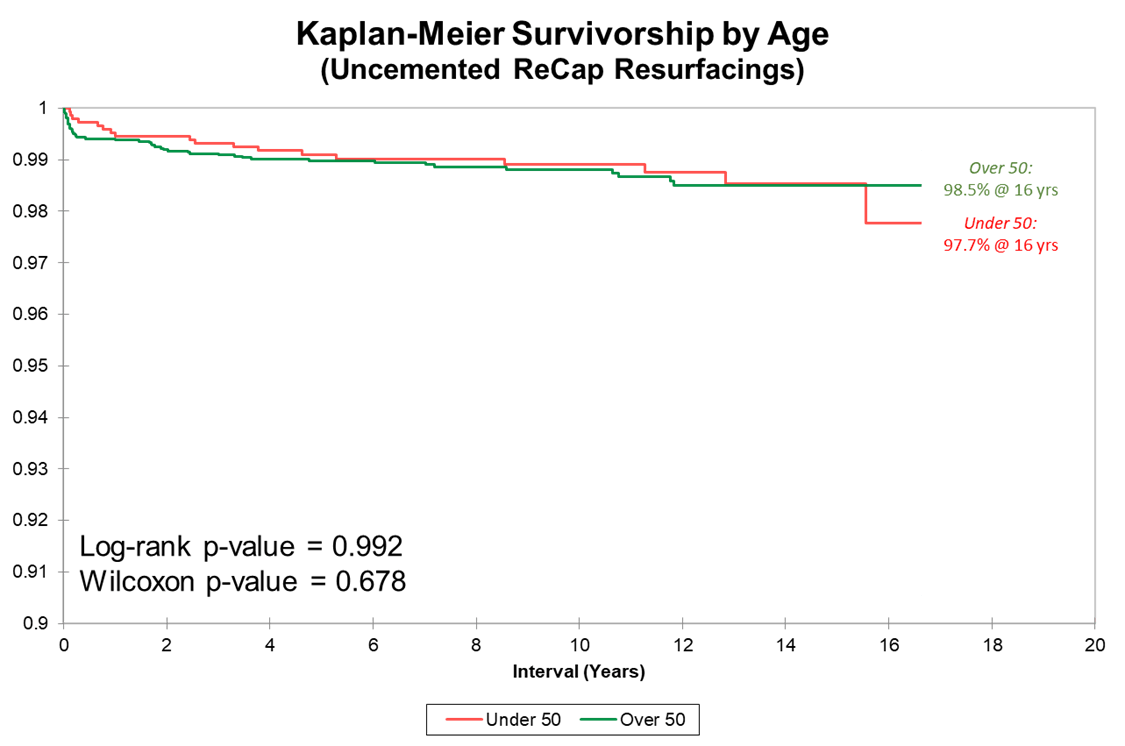
Above is the survivorship curve separated by age group for our uncemented ReCap group. Note the y-axis starts at 90%.
There is no difference in survivorship or raw failure rate based on age, unlike the typical pattern found at many other surgery centers.
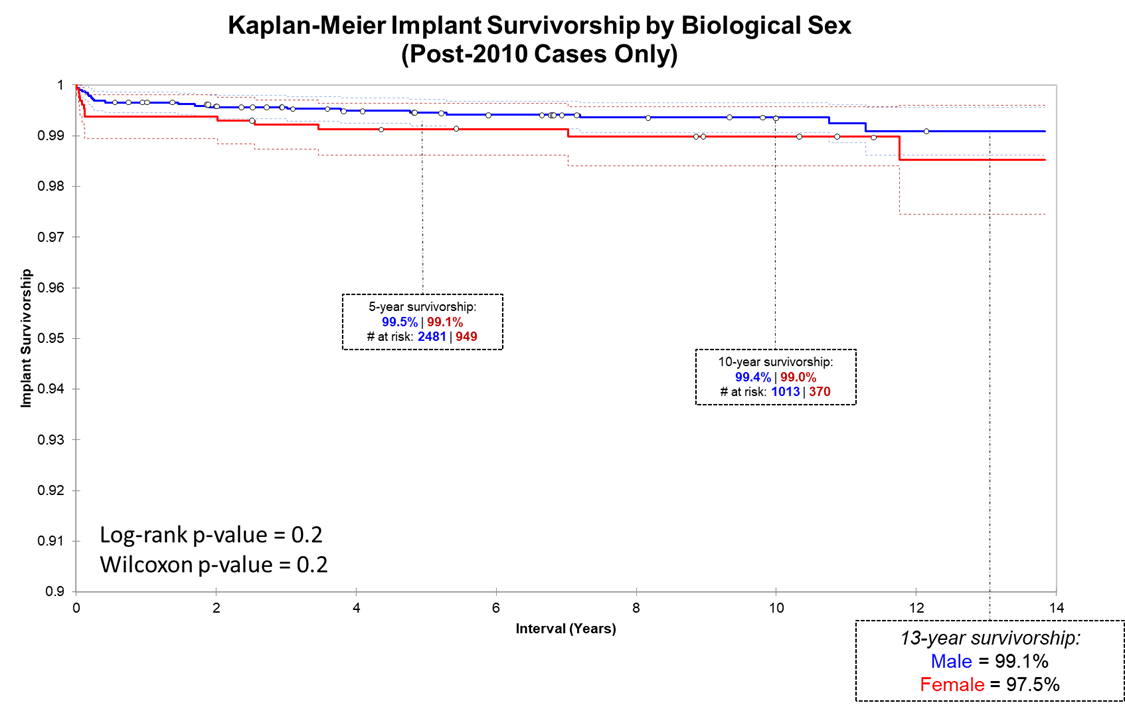
Many orthopedic surgeons exclude women from HRA because of poor published results. We, however, elected to investigate WHY implants in women were underperforming and to adjust implant design + surgical technique rather than exclude women from surgery. After the implementation of new protocols from 2007-2009, implant survivorship between men and women is no longer significantly different.
The implant survivorship data reported here far surpasses joint implant registry data from Britain, Sweden, and Australia (for both THR and HRA) where these types of data are kept. These are publicly available, and you can get access them online for free. Registry data can be thought of as average surgeon implant survivorship for purposes of a benchmark. But the most important factor in the outcome of any operation is individual surgeon skill. It is hard to know at which level a surgeon you are considering can perform. Anecdotal reports from a few patients or reputation are a poor substitute for data. Few surgeons provide written data such as I do.
Remember, implant survivorship is not the only factor that needs to be considered in deciding between THR and HRA. Other proven advantages of HRA include better functional outcomes, less residual thigh pain, fewer dislocations, bone preservation, and longer life expectancy.
Among Dr. Gross’ patient cohort, 98.7% report being satisfied with their surgery at 2 years postoperative. After all revisions, reoperations, and complications are accounted for, there are still approximately 3.4% of patients who experience moderate unexplained residual pain after HRA. The risk of moderate residual unexplained pain in THR is 20%. This means we cannot determine a specific reason why they are not satisfied. Some may have referred pain from their back, sacroiliac joint, or from soft tissue problems we are unable to diagnose.
In a THR thigh pain from the stem is a common cause of residual pain. Residual pain is a highly subjective data point. It may just represent the fact that HRA does not result in a normal hip. One person’s insignificant pain may be graded as moderate by another. If we can’t diagnose a cause of pain, we don’t recommend revision surgery for “unexplained” pain. If a revision is still performed, sometimes a patient improves, but most often they subject themselves to the risk of revision surgery and do not improve. There is no measurable difference in the speed of recovery between THR and HRA.
Since 2007 Dr. Gross has used primarily the Biomet Recap / Magnum uncemented metal-on-metal hip resurfacing system. The majority of the data presented here is for this system. The FDA has approved these implants for sale in the US. They are however NOT approved for use as a total hip resurfacing combination. Dr. Gross uses them for this “off-label” purpose.
The FDA regulates implant companies. The FDA does not regulate doctors. Once an implant is approved for sale, it can be used for any purpose that a doctor feels is best. When an implant company gets FDA approval for an implant, the company may only market and promote this implant for the “indication” that they have received from the FDA.
This is true even if there are scientific papers that demonstrate that this implant is safe and effective when used differently. Companies don’t generally want to spend the effort and expense to gain new “indications” from the FDA because doctors can already use them however they like and doctors generally rely on their own experience as well as scientific publication by their peers, rather than FDA guidelines. There are many examples of drugs and implants whose primary current use is not the original FDA “indication”. Basically, the FDA regulates drug and implant companies conduct, but has no jurisdiction over doctors. We have the education, training, and experience to use an implant or drug for whatever purpose we think is best.
This is a perfectly legal and common practice. I am not even required to disclose off-label use to patients. I chose to do so because metal-metal resurfacing is a highly controversial practice. I use the Biomet Recap/Magnum in an off-label fashion and have the best implant survivorship in the published literature. If you prefer a device that is FDA “indicated” for metal-metal resurfacing, I would be happy to use the Smith-Nephew Birmingham Hip Resurfacing (BHR) device for you. It is the most used hip resurfacing device in the world market and has been studied the most.
But I can only implant this in men with a bearing size of greater than 48mm. These fit most men. Smaller sizes have been removed from the market and the manufacturer has placed a warning on the label against use in women. I have excellent outcomes in smaller-sized implants (with the Biomet system which is very similar in design) and in women and completely disagree with the manufacturer on this topic.
However, they simply do not supply the smaller sizes any longer. Even in women who need larger sizes, the manufacturer's labeling makes me unwilling to risk this due to our overly litigious country. In my opinion, the BHR has similar outcomes at 10 years in men with OA and good bone. Because of the cemented femoral fixation, it does not do as well in anyone with weak bone or bone defects (osteonecrosis, cysts) on the femoral side.
Past results do not guarantee future complication rates. Although the above represent the most common complications associated with this procedure, others could also occur. We continue to strive to make improvements and hope that these complication rates can be further decreased as we gain even more experience.
- Dr. Gross is the operating surgeon (No trainee will perform your operation).
- Dr. Gross developed the Biomet implants but no longer receives royalties for these implants.
- Biomet Recap and Magnum components are FDA-approved. Use as a total hip resurfacing is however considered off-label.
- Information from your treatment is used for research purposes, but you will not be identified.
If you have any questions about the above information, please don’t hesitate to ask.
2. Total Hip Replacement (THR)
The need for hip replacement continues to shrink as the complication rate for resurfacing falls. Hip resurfacing started as a temporizing measure for younger patients to preserve bone. Most surgeons still prefer plastic-bearing hip replacement to hip resurfacing. My first choice is usually hip resurfacing. In the few patients that are not good resurfacing candidates, my next choice used to be large metal bearing total hip replacement. Hip dislocations are completely eliminated by this choice
Other surgeons are reluctant to use these implants because of a fear of adverse metal wear-related failure (AWRF). This has been a common failure mode among some brands (DePuy ASR recall 2010). But this is a rare problem with the Biomet design. Because of decreased demand for large metal-bearing THR, Zimmer-Biomet discontinued the sale of this implant several years ago. I now use the best alternative which is a dual mobility ceramic/polyethylene bearing which is nearly as good.
With the large metal-bearing Biomet Magnum THR, I have a 97% 15-year implant survivorship (for patients average age of 60) with no dislocations, which far surpasses registry benchmarks (approximately 92% 10-year survivorship for a similar age group). Also, a standard total hip carries a 3% dislocation risk and a 1-5% trunion corrosion and requires permanent restrictions. I generally perform hip replacement in the very obese (BMI > 35), patients older than 70 years, those with severe osteoporosis, or severe bone deformities.
Failures in 211 cases – TOTAL: 2.8% raw failure rate 16 years postoperative
-
Failure
-
# cases
- Failure of acetabular ingrowth
- 2
- Trunion corrosion
- 2
- AWRF due to acetabular malposition
- 1
- Late infection
- 1
3. Revision of Hip Resurfacing
More complicated than primary surgery. Our results are very close to those of our primary resurfacing cases with a 96% 8-year implant survivorship. Our most problematic group is revision for loose acetabular components. Other surgeons have had extremely poor results in revision for adverse wear-related failures (AWRF). Using an approach of limited debridement and repositioning of new metal-bearing acetabular components in more ideal inclination angles, we have had a 100% success rate in this problematic group.

